Human cell becomes living laser - Scientists have for the first time created laser light using living biological material: a single human cell and some jellyfish protein.
"Lasers started from physics and are viewed as engineering devices," says Seok-Hyun Yun, an optical physicist at Harvard Medical School and Massachusetts General Hospital in Boston, who created the 'living laser' with his colleague Malte Gather. "This is the first time that we have used biological materials to build a laser and generate light from something that is living." The finding is reported today in Nature Photonics 1.
Building a laser requires two things: a lasing material that amplifies light from an external source (a 'gain medium') and an arrangement of mirrors (an 'optical cavity'), which concentrates and aligns the light waves into a tight beam. Until now, the gain medium has only been made from non-biological substances such as doped crystals, semiconductors or gases, but in this case the researchers used enhanced green fluorescent protein (GFP) — the substance that makes jellyfish bioluminescent, which is used extensively in cell biology to label cells.
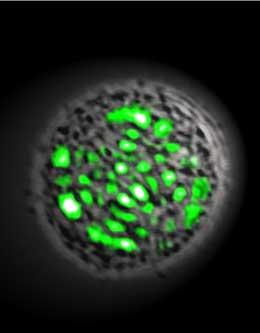
Microscope image of a living laser in action. Due to the irregular internal structure of the cell, the laser spot has an apparently random pattern.
The team engineered human embryonic kidney cells to produce GFP, then placed a single cell between two mirrors to make an optical cavity just 20 micrometres across. When they fed the cell pulses of blue light, it emitted a directional laser beam visible with the naked eye — and the cell wasn't harmed.
The width of the laser beam is "tiny" and "fairly weak" in its brightness compared to traditional lasers, says Yun, but "an order of magnitude" brighter than natural jellyfish fluorescence, with a "beautiful green" colour.
Illuminating biology
Yun and Gather have some broad and speculative ideas about how the technology might be used.
They suggest that biologists could turn cells of interest into lasers to study them. The light produced has a unique emission spectrum related to both the structure of the cell and the proteins inside it. "By analysing the pattern you can get some idea of what is happening inside the cell," says Yun.
The researchers also suggest possible medical applications. Doctors today shine lasers into the body to gather images or to treat disease by attacking cells. Yun thinks that lasers could instead be generated or amplified inside the body, where they could penetrate the relevant tissues more deeply. But more work is needed first — including developing the laser so that it works inside an actual living organism. To achieve this, Yun envisages integrating a nano-scale optical cavity into the laser cell itself. Technologies to make such cavities are emerging, he says, and once they are available they could be used to create a cell that could "self lase" from inside tissue.
Experts praise the work as interesting and creative. "It is kind of neat," says Michael Berns, a biomedical engineer at the University of California, Irvine. "I have been working on cells and lasers for 40 years, and I don't think I would have thought of this."
But he says that the technique might more feasibly be used to study individual cells than for medical applications. He points out that external light is needed to stimulate the laser action, which would be difficult in the body, potentially limiting the technique to thin-tissue systems or cells in culture or suspension. ( nature.com )
Blog : Brilliant Purple | Human cell becomes living laser
"Lasers started from physics and are viewed as engineering devices," says Seok-Hyun Yun, an optical physicist at Harvard Medical School and Massachusetts General Hospital in Boston, who created the 'living laser' with his colleague Malte Gather. "This is the first time that we have used biological materials to build a laser and generate light from something that is living." The finding is reported today in Nature Photonics 1.
Building a laser requires two things: a lasing material that amplifies light from an external source (a 'gain medium') and an arrangement of mirrors (an 'optical cavity'), which concentrates and aligns the light waves into a tight beam. Until now, the gain medium has only been made from non-biological substances such as doped crystals, semiconductors or gases, but in this case the researchers used enhanced green fluorescent protein (GFP) — the substance that makes jellyfish bioluminescent, which is used extensively in cell biology to label cells.

Microscope image of a living laser in action. Due to the irregular internal structure of the cell, the laser spot has an apparently random pattern.
The team engineered human embryonic kidney cells to produce GFP, then placed a single cell between two mirrors to make an optical cavity just 20 micrometres across. When they fed the cell pulses of blue light, it emitted a directional laser beam visible with the naked eye — and the cell wasn't harmed.
The width of the laser beam is "tiny" and "fairly weak" in its brightness compared to traditional lasers, says Yun, but "an order of magnitude" brighter than natural jellyfish fluorescence, with a "beautiful green" colour.
Illuminating biology
Yun and Gather have some broad and speculative ideas about how the technology might be used.
They suggest that biologists could turn cells of interest into lasers to study them. The light produced has a unique emission spectrum related to both the structure of the cell and the proteins inside it. "By analysing the pattern you can get some idea of what is happening inside the cell," says Yun.
The researchers also suggest possible medical applications. Doctors today shine lasers into the body to gather images or to treat disease by attacking cells. Yun thinks that lasers could instead be generated or amplified inside the body, where they could penetrate the relevant tissues more deeply. But more work is needed first — including developing the laser so that it works inside an actual living organism. To achieve this, Yun envisages integrating a nano-scale optical cavity into the laser cell itself. Technologies to make such cavities are emerging, he says, and once they are available they could be used to create a cell that could "self lase" from inside tissue.
Experts praise the work as interesting and creative. "It is kind of neat," says Michael Berns, a biomedical engineer at the University of California, Irvine. "I have been working on cells and lasers for 40 years, and I don't think I would have thought of this."
But he says that the technique might more feasibly be used to study individual cells than for medical applications. He points out that external light is needed to stimulate the laser action, which would be difficult in the body, potentially limiting the technique to thin-tissue systems or cells in culture or suspension. ( nature.com )
No comments:
Post a Comment